Parkinson’s Disease Physiotherapy
Parkinson’s disease affects movement and other functions of the brain. Understanding its symptoms, diagnosis, and treatment will let you take an active role in caring for yourself or a loved one
It’s clear that exercise helps people with early- and middle-stage Parkinson’s disease. What isn’t clear is exactly what type of exercise helps people with this illness. It’s also unclear what intensity of exercise helps.
Recently, researchers have taken great interest in exercise as a treatment for Parkinson’s disease. Traditionally, Parkinson’s disease has been treated using medications and surgery; however, exercise is a low-cost, noninvasive intervention with few negative side effects other than minor aches and pains. Moreover, the efficacy of drugs used to treat Parkinson’s disease decreases over time, and disease-modifying non-pharmacologic interventions are direly needed to combat the illness.
Before we look at a couple of studies examining Parkinson’s disease exercises, it’s important to clarify one point. It may seem counterintuitive for a person with Parkinson’s disease to engage in high-intensity exercise on a treadmill. After all, Parkinson’s disease is a neurodegenerative condition that results in rigidity, tremor, gait instability, and so forth. But keep in mind that the patients in these studies were earlier along in their disease trajectory. In other words, high-intensity exercise wasn’t tested on people with late-stage Parkinson’s disease.
Parkinson’s Disease: Background Info
Parkinson’s disease usually occurs spontaneously and is of unknown origin. About one million Americans live with Parkinson’s disease. Worldwide there are 10 million people living with Parkinson’s disease. The average age of diagnosis of those with Parkinson’s disease is 60 years, and the disease gradually progresses during the next 10 to 25 years after diagnosis.
In the brain, nerve cells use dopamine to control muscle movements. In people with Parkinson’s disease, the brain cells making dopamine gradually die. Over time, it becomes harder for people with Parkinson’s disease to move their muscles.
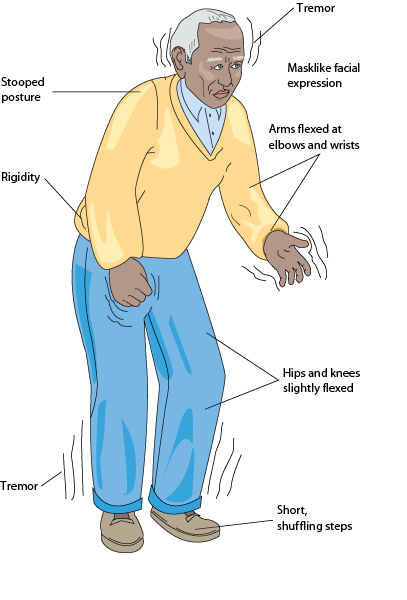
The following are some symptoms of Parkinson’s disease:
· “Pill-rolling” tremor of the hands at rest
· Slow muscle movements (i.e., bradykinesia)
· Drooling
· Shuffling gait
· Anxiety
· Monotone voice
· Stooped posture
· Constipation
· Cognitive impairment
· Restlessness
The diagnosis of Parkinson’s disease is based on history and physical examination findings. Importantly, neuroimaging, EEG, and spinal fluid studies are usually within normal limits for age in those with Parkinson’s disease.
Unfortunately, there is no cure for Parkinson’s disease. Certain drugs such as carbidopa-levodopa (Sinemet) and MAO-B inhibitors can be used to substitute or increase dopamine levels in the brain. These dopaminergic drugs, however, lose efficacy over time and have negative side effects.
Parkinson’s disease is also treated symptomatically with drugs that help with mood disturbances, pain complaints, and sleep problems.
Deep-brain stimulation is a type of surgery used to treat Parkinson’s disease. This procedure can help with disabling neurological symptoms, such as tremor, rigidity, stiffness, and problems with walking.
In 2001, results from a Cochrane Review suggested that there was insufficient evidence to either support or refute the benefit of any specific exercise in the treatment of Parkinson’s disease. Moreover, at that time, in experimental settings, the effects of exercise on Parkinson’s disease were short-term, with no long-term follow-up. Nevertheless, for years it has been assumed that ongoing exercise in those with Parkinson’s disease was necessary to slow declines in strength, flexibility, and balance.
Endurance exercises have been shown to promote the growth and development of nerves and protect nerve cells in animal models. However, animal models are not the same as humans.
Finally, a number of retrospective studies have shown that moderate to vigorous exercise during midlife can protect against Parkinson’s disease in later life.
Long-Term Response to Exercise
In November 2012, Schenkman and colleagues examined the short- and long-term benefits of two different types of exercise in study participants with Parkinson’s disease. The randomized controlled exercise intervention trial occurred during a period of 16 months and was conducted in outpatient clinics.
In the study, 121 participants with either early- or mid-stage Parkinson’s disease were assigned to one of three groups. The first group engaged in flexibility/balance/function exercises. The second group engaged in aerobic exercise using a treadmill, bike, or elliptical trainer. The third, or control group, exercised at home—as outlined in a fitness program called Fitness Counts, which was developed by the National Parkinson Foundation.
The first two groups were supervised while exercising three times a week for four months. Thereafter, supervision was tapered to once a month for the duration of the 16-month study. The control group was supervised once per month for 16 months.
Participants were evaluated using various tests at 4, 10, and 16 months. Here are the researchers’ findings:
· At four months, overall function improved in the flexibility/balance/function group compared with that of the aerobic exercise and control groups.
· At 4, 10, and 16 months, walking economy (i.e., movement efficiency) improved in the aerobic exercise group as compared with that of the flexibility/balance/function group.
· Balance was the same among all groups.
· At 4 and 16 months, activities of daily living improved in the flexibility/balance/function group as compared with that of the control group.
The results of this study suggest that different types of exercises confer different benefits for those with Parkinson’s disease. Endurance programs seem to proffer the greatest long-term benefits.
According to Schenkman and co-authors:
“Qualitative reports from graduates of the 16-month study emphasize that people need ongoing support to maintain regular exercise. We strongly recommend that clinicians find ways to assist individuals with PD [Parkinson’s disease] to develop and maintain long-term exercise habits, including appropriate exercise programs as well as continued re-evaluation and support.”
Of note, this study did have its limitations.
First, the control group engaged in some exercise because it would be unethical for these participants not to receive any exercise at all. In other words, although a “true” control group would not engage in exercise during 16 months, recommending this option would be detrimental to health. According to the researchers, overall the Fitness Counts guidance issued by the National Parkinson Foundation did result in some benefit, but not as much benefit as that experienced by participants in supervised exercise programs involving either flexibility/balance/function exercises or aerobic exercise.
Second, this study was conducted in Colorado, which is one of the fittest states in the Union. It’s likely that participants in this study exercised more at baseline than do people in other states thus making results less generalizable.
Third, participants in each of the three groups received different amounts of individualized attention, which could confound results.
Finally, it was difficult to assess adherence to exercise regimens, and researchers relied on activity logs—not activity monitors—to make such determinations
High-Intensity Exercise and Parkinson’s Disease
The Study in Parkinson Disease of Exercise (SPARX) was a phase 2, randomized clinical trial conducted by Schenkman and colleagues between May 2012 and November 2015. Participants in the trial were evaluated after six months.
In the SPARX trial, 128 participants with Parkinson’s disease who were aged between 40 and 80 years were divided into three groups.
The first experimental group underwent high-intensity exercise, the second experimental group underwent moderate-intensity exercise, and members of the control group were waitlisted for future exercise intervention. (Again, it would be unethical to deny the control group the opportunity to exercise.)
Of note, the participants in the study were diagnosed with de novo Parkinson’s disease (i.e., diagnosed within the previous five years) and were not expected to need dopaminergic (antiparkinson) medications during the six-month duration of their participation. Furthermore, none of the participants were previously engaged in moderate- or high-intensity exercise.
High-intensity exercise consisted of four days per week on the treadmill at 80 percent to 85 percent maximal heart rate. Moderate-intensity exercise also occurred four times a week but at between 60 percent and 65 percent maximal heart rate.
The aim of the phase 2 SPARX trial was to determine whether patients with Parkinson’s disease could safely engage in high-intensity exercise. The researchers didn’t determine whether exercising at between 80 percent and 85 percent heart rate intensity actually resulted in clinical benefit for those with de novo Parkinson disease. Ultimately, the researchers were interested in determining whether high-intensity exercise could be tested in phase 3 trials. These phase 3 trials would then examine the possible benefits of this intervention.
According to Schenkman and co-authors:
“One of the limiting factors to moving to phase 3 trials is that the appropriate dose of exercise has yet to be established for any exercise modality. Exercise imposes a substantial participant commitment of time and effort compared with pharmacologic interventions. The futility design was used to specifically establish whether further study of specific exercise dose is warranted, proving a method to efficiently determine the appropriate dose before moving forward to the first phase 3 exercise trial in Parkinson disease. Findings of nonfutility of high-intensity treadmill exercise should move the field forward substantially.”
The SPARX study did have limitations.
First, high-intensity exercise was performed only on a treadmill and not using other types of exercise equipment.
Second, both treadmill speed and intensity were adjusted to yield high-intensity exercise; however, it’s unclear whether either or both of these variables could improve motor symptoms in Parkinson’s disease.
Third, it’s unclear how combining high-intensity treadmill exercise with other physiotherapy interventions with known benefit for those with Parkinson’s disease, such as Tai Chi or strength training, could result in even greater clinical benefit.
NOTE
We know that exercise helps people with Parkinson’s disease. New research suggests that high-intensity treadmill exercise may be prescribed safely for patients with mild Parkinson’s disease and that people with early- to mid-stage Parkinson’s disease benefit from different types of exercises, including flexibility, balance, and aerobic.
More research needs to be done to figure out the exact benefits of such high-intensity exercise. If you or a loved one are diagnosed with Parkinson’s disease, please consult with your physiotherapist.